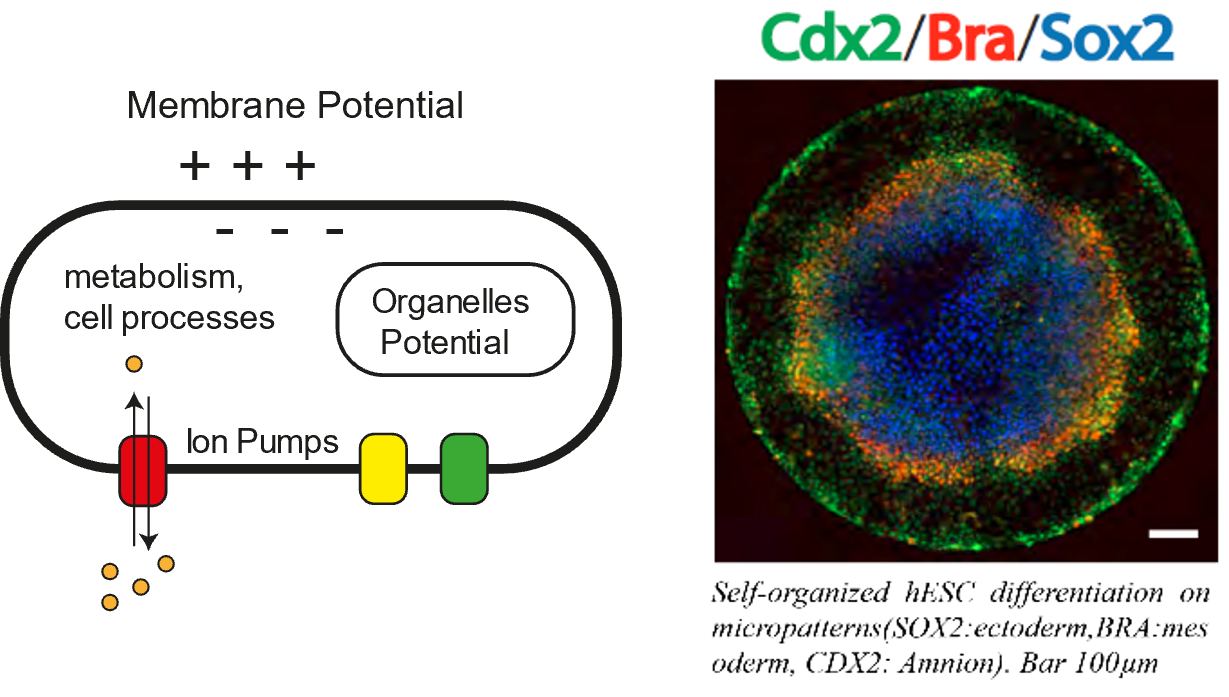
All cell membranes present an electric potential. This is called the Resting Membrane Potential (RMP) and is the trace of the difference of ionic charges between the extracellular and the intracellular spaces. This is very well known in the so-called excitable cells (e.g. neurons, muscles) which can change their electric potential thanks to ion channels regulation. In the past years however there has been a regain of interest on the role of the membrane potential in participating to biological regulation at the scale of the cell and the tissue in non-neural cells. There is now a clear set of evidence that membrane voltage is involved in / correlates with non-neural cell regulation in a variety of situations from embryonic development to cancer1 [1].
Among several striking facts, stable gradients of RMP at the scale of the embryo of model organisms have been observed. These gradients seem to provide positional information much like classical morphogen gradients and appeared to be crucial to establish the body plan23[2, 3]. The fact that Embryonic Stem cells are depolarized and become hyperpolarized as they differentiate and that RMP modulates developmentally relevant signaling pathways suggest that it could also play a role in Human development4 [1, 4]. To test this hypothesis, we propose to use Human Embryonic Stem Cells (hESC) cultured as a monolayer on adhesive circular micro-patterns. This in vitro system recapitulates faithfully what is known of the dynamics of gene expression and key morphogenetic features of the gastrula and is fully accessible to manipulation and observation.
The goal of this internship is to use recently developed Genetically Encoded Voltage Indicators (GEVI) to observe RMP in live micropatterned hESC colonies in order to assess if the spatio-temporal variation of RMP (From depolarized stem cells to hyperpolarized differentiated cells) correlates with that of the expression of genetic markers of cell differentiation in hESC colonies. We will also perturb RMP chemical inhibitors and/or optogenetic tools to ask if it plays a causal role in cell fate commitment.
- Bates E (2015) Ion Channels in Development and Cancer. Annu Rev Cell Dev Biol 31:231–247 . https://doi.org/10.1146/annurev-cellbio-100814-125338 ↩︎
- Levin M (2021) Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 184:1971–1989 . https://doi.org/10.1016/j.cell.2021.02.034 ↩︎
- Adams DS, Levin M (2013) Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res 352:95–122. https://doi.org/10.1007/s00441-012-1329-4 ↩︎
- Binggeli R, Weinstein RC (1986) Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. Journal of Theoretical Biology 123:377–401 . https://doi.org/10.1016/S0022-5193(86)80209-0 ↩︎